The major industrial source of hydrogen gas is the reaction of methane and water at high temperatures (800 - 1000°C) and high pressures (10 - 15 atm) with nickel as a catalyst. CH4(g) + H2O(g) 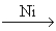 CO2(g) + 3H2(g)
CO2(g) + 3H2(g)
If 171.5 g of CH4 and 171.5 g of H2O are reacted at 945°C and 11.0 atm,how much hydrogen should be available for industrial use?
Definitions:
Historical Data
Previous data collected over time that is used to analyze trends, forecast future events, or make comparisons.
Delineated List
A detailed enumeration or cataloging of items, often structured for clarity and ease of understanding.
Request For Proposal
A document issued by an organization to solicit proposals from potential suppliers for a desired project or service.
Bidding Process
The procedure by which a contractor is selected to work on a project, usually involving submission of proposals and selection based on criteria such as price and capability.
Q8: Which of the following result(s)in an
Q18: Which of the following statements concerning
Q22: What are the building blocks of proteins?<br>A)nucleotides<br>B)glucose
Q40: A radioactive sample has an initial
Q40: Which of the following materials is added
Q46: In the following nuclear equation,identify the
Q65: The complex FeL<sub>6</sub><sup>2+</sup>,where L is a neutral
Q68: The decomposition of nitric acid in sunlight
Q96: Ionic hydrides are formed with hydrogen combined
Q127: Using the following data to calculate