The major industrial source of hydrogen gas is the reaction of methane and water at high temperatures (800 - 1000°C) and high pressures (10 - 15 atm) with nickel as a catalyst. CH4(g) + H2O(g) 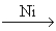 CO2(g) + 3H2(g)
CO2(g) + 3H2(g)
If 171.5 g of CH4 and 171.5 g of H2O are reacted at 945°C and 11.0 atm,how much hydrogen should be available for industrial use?
Definitions:
True Probabilities
The actual likelihood of all possible outcomes of a random event, often contrasted with estimated probabilities based on models or historical data.
Rates Of Return
The gains from an investment over a specific period, often measured as a percentage of the investment's initial cost. This represents the profitability of an investment.
Recent Experience
This term refers to the latest available data or outcomes from events, often used to analyze current trends or performance.
Prior Beliefs
The pre-existing beliefs or expectations an individual holds before receiving new information or evidence.
Q9: Give (in order)the correct coefficients to
Q11: Copper(I)complexes would be expected to be colorless.
Q29: How many oxygen atoms are there in
Q48: For the reaction P<sub>4</sub>O<sub>10</sub>(s)+ 6H<sub>2</sub>O(l) <span
Q49: hydration of 2-butene
Q75: Consider the following processes:<br>I.condensation of a
Q105: When 233.1 g of ethylene (C<sub>2</sub>H<sub>4</sub>)burns in
Q105: Choose the species with the largest ionization
Q126: Teflon is an example of a<br>A)copolymer<br>B)homopolymer<br>C)dimer<br>D)two of
Q160: What carbohydrate breaks down rapidly when energy