One of the hopes for solving the world's energy problem is to make use of the fusion reaction: 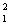 H +
H + 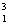 H
H 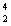 He +
He + 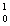 n + energy How much energy is released when one mole of deuterium is fused with one mole of tritium according to the above reaction? The masses of the atoms and the neutrons are:
n + energy How much energy is released when one mole of deuterium is fused with one mole of tritium according to the above reaction? The masses of the atoms and the neutrons are: 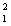 H = 2.0140 amu
H = 2.0140 amu 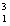 H = 3.01605 amu
H = 3.01605 amu 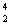 He = 4.002603 amu
He = 4.002603 amu 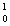 n = 1.008665 amu
n = 1.008665 amu
The speed of light is 2.9979 108 m/s
Definitions:
Holder In Due Course
A legal term for a party that has acquired a negotiable instrument in good faith and for value, and thus has certain protections against defects in title and prior claims.
Consideration
The value (such as money, service, or goods) that is exchanged between parties in a contract, making the agreement legally binding.
Value
The importance, worth, or usefulness of something, often in terms of money, social benefits, or personal significance.
Shelter Principle
A legal rule in negotiable instruments law that allows a person who obtains a negotiable instrument under certain conditions to acquire greater rights than the transferor had.
Q7: Which statement is always true of the
Q12: Why do transition metals show a lot
Q15: Which model(s)accounts for the magnetism and color
Q18: Which of the following is not a
Q32: As atomic mass increases,the proton/neutron ratio of
Q33: The equilibrium constant of a certain
Q48: Solubility Products (K<sub>sp</sub>) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="Solubility Products
Q56: The concentration of Mg<sup>2+</sup> in seawater
Q72: Which group contains two elements that exhibit
Q123: Consider the two amino acids below.The circled