The following question refers to a galvanic cell that utilizes the following reaction (unbalanced) : (AuCl4) -(aq) + Cu(s) Au(s) + Cl-(aq) + Cu2+(aq)
Given the following information,determine the standard cell potential:
Species
Standard Reduction Potential (V)
Au3+(aq)
1.4980
Cu2+(aq) 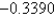
Definitions:
Statement Of Cash Flows
A financial statement that consolidates information about all the money a company gets from its regular operations and outside investments, alongside the money spent on business operations and investments within a certain timeframe.
Investing Activities
Part of a company's cash flow statement that shows the amount of money spent or generated from various investment-related activities in non-current assets.
Contingent Activities
Actions or outcomes that are possible and might affect a project or transaction but their occurrence is dependent on future events.
Noncash Investing
Investing activities that do not involve immediate cash transactions, such as acquiring assets through the issuance of equity or exchanging one asset for another.
Q11: Which of the following is not a
Q22: What is <span class="ql-formula" data-value="\Delta"><span
Q36: You have solutions of 0.200 M
Q47: The element with the widest liquid range
Q56: The concentration of Mg<sup>2+</sup> in seawater
Q63: Which of the following is incorrectly named?<br>A)Pb(NO<sub>3</sub>)<sub>2</sub>,lead(II)nitrate<br>B)NH<sub>4</sub>ClO<sub>4</sub>,ammonium
Q76: dichlorine heptoxide
Q77: The most likely decay mode (or
Q77: What is the maximum concentration of
Q122: A monoprotic weak acid when dissolved