Multiple Choice
Choose the correct statement given the following information: 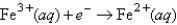
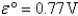
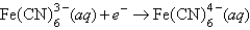
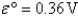
Definitions:
Related Questions
Q3: In the qualitative analysis scheme for metal
Q6: <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow><mi mathvariant="normal">Δ</mi></mrow><annotation
Q35: For this sample the ratio of the
Q38: The solubility of silver phosphate,Ag<sub>3</sub>PO<sub>4</sub>,at 25°C
Q43: Alkaline earth metals react less vigorously with
Q54: Consider the titration of 100.0 mL
Q59: Choose the metal with the largest first
Q79: Three samples of a solid substance composed
Q134: In cation-exchange resins,what ion replaces Ca<sup>2+</sup> and
Q145: What reason is given for the stability