The following has a potential of 0.92 V: 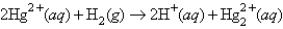 If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then for the half-reaction
If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then for the half-reaction 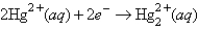 would be
would be
Definitions:
Inventory Turnover
A metric representing the frequency at which a business's stock is sold and restocked within a given timeframe, reflecting how effectively inventory is handled.
Current Ratio
An indicator of how well a company can handle its immediate debts, assessing its ability to cover obligations maturing in one year or less.
Acid-test Ratio
A financial metric that measures a company's ability to cover its short-term liabilities with its most liquid assets, excluding inventory.
Debt-to-equity Ratio
The debt-to-equity ratio is a financial leverage ratio that compares a company's total liabilities to its shareholder equity, indicating how much the company is financing its operations through debt versus wholly owned funds.
Q2: Consider a certain type of nucleus that
Q12: Consider a solution containing the following cations:
Q14: Choose the element obtained from liquification of
Q26: A chemical reaction is most likely to
Q46: The chemistry of silicon is dominated by
Q52: A 0.20-mL sample of a solution
Q74: Electron capture transforms <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="Electron capture
Q84: phosphoric acid
Q112: If you could increase the concentration of
Q139: What nitrogen-containing compound is used as rocket