Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment) : 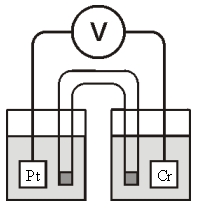 0.10 M MnO4- 0.40 M Cr3+ 0.20 M Mn2+ 0.30 M Cr2O72-
0.10 M MnO4- 0.40 M Cr3+ 0.20 M Mn2+ 0.30 M Cr2O72-
0.010 M H+ 0.010 M H+
The standard reduction potentials are as follows:
MnO4- + 8H+ + 5e- Mn2+ + 4H2O, = 1.506 V
Cr2O72- + 14H+ + 6e- 2Cr3+ + 7H2O, = 1.330 V
What is the value of cell?
Definitions:
Comorbidity
The occurrence of one or more secondary diseases or conditions alongside a main disease or condition.
Overlap
The similarity of symptoms in two or more different disorders (that is, some of the same criteria apply to different diagnoses), which creates problems with diagnosis. See also comorbidity.
Personality Profiles
Descriptions of an individual's characteristics, traits, or tendencies as they relate to their personality.
Personality Disorder
A type of mental disorder characterized by enduring maladaptive patterns of behavior, cognition, and inner experience.
Q12: The pH of a 0.118 M
Q42: Which of the following is a major
Q46: In the crystal field model,ligands are treated
Q51: Given that <span class="ql-formula" data-value="\Delta"><span
Q57: In the titration of a weak acid
Q58: At 699 K, <span class="ql-formula" data-value="\Delta"><span
Q80: The concentration of Ag(NH<sub>3</sub>)<sub>2</sub><sup>+</sup> at equilibrium
Q89: Which of the following will not produce
Q90: The complex ions of Zn<sup>2+</sup> are all
Q107: Given: 2H<sup>+</sup>(aq)+ 2e<sup>-</sup> <span class="ql-formula"