Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment) : 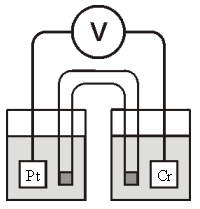 0.10 M MnO4- 0.40 M Cr3+
0.10 M MnO4- 0.40 M Cr3+
0.20 M Mn2+ 0.30 M Cr2O72-
0.010 M H+ 0.010 M H+
The standard reduction potentials are as follows:
MnO4- + 8H+ + 5e- Mn2+ + 4H2O, = 1.51 V
Cr2O72- + 14H+ + 6e- 2Cr3+ + 7H2O, =1.33 V
-When current is allowed to flow,which species is reduced?
Definitions:
Preponderance of the Evidence
A standard of proof in civil cases, requiring that a party's evidence is more convincing than the opponent's evidence to win the case.
Advisory
Providing suggestions, recommendations, or guidance, often from a position of expertise.
Mandatory
Required by law or rules; obligatory, leaving no room for personal discretion or choice.
Massive Overstock
Describes a situation where a retailer or inventory-based business holds a significantly larger quantity of products than needed or demanded, leading to excess stock.
Q17: It is desired to determine the
Q22: Metals usually have higher melting points than
Q22: Which of the following compounds has
Q30: Consider the reaction 2NO<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg"
Q59: Choose the metal with the largest first
Q63: Choose the metal that reacts least vigorously
Q68: The decomposition of nitric acid in sunlight
Q69: Choose the element whose ion has the
Q86: The dihydrogenphosphate ion,H<sub>2</sub>PO<sub>4</sub><sup>-</sup>,has both a conjugate acid
Q112: You have 100.0 mL of a solution