Determine the standard potential, ,of a cell that employs the reaction: Fe + Cu2+ Cu + Fe2+.
Reaction
(volts) 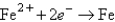 -0.441
-0.441 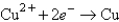 +0.340
+0.340
Definitions:
Minority Students
Students who are part of a racial, ethnic, cultural, or socioeconomic group that is underrepresented or marginalized in a specific context.
Margin of Error
An expression of the amount of random sampling error in a survey's results, indicating a range within which the true value lies with a certain probability.
Sample Size
The number of observations or replicates included in a statistical sample, affecting the precision and reliability of its estimates.
Largest Sample
The most extensive or most significant sample size from a set of samples collected for statistical analysis or experimentation.
Q15: The solubility of CaSO<sub>4</sub> in pure water
Q50: Which of the following oxides is amphoteric?<br>A)BeO<br>B)MgO<br>C)CaO<br>D)SrO<br>E)BaO
Q52: A 0.20-mL sample of a solution
Q70: The following question refers to the
Q77: A titration of 200.0 mL of
Q91: Determine <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q104: Gold (atomic mass = 197.0)is plated from
Q126: Which of the following is true for
Q130: The ability of the Group 5A elements
Q138: The reduction potentials for Au<sup>3+</sup> and