The following has a potential of 0.92 V: 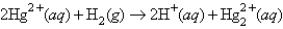 If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then for the half-reaction
If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then for the half-reaction 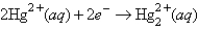 would be
would be
Definitions:
Wild Card
In computing, a symbol used to substitute for any other character or characters in a string.
Third-Party Verification
An independent assessment performed by an external entity to verify the claims made by a party, often used in transactions or compliance checks.
Debit Card
A purchasing card that immediately takes money out of an individual's bank account to settle a transaction.
Physical Store Locations
Brick-and-mortar sites where businesses sell their products or offer services directly to customers in person.
Q7: Gallium metal with I<sub>2</sub>(s).
Q18: Which of the following is not a
Q25: In which of the following cases
Q34: Calculate the molar concentration of uncomplexed
Q77: Which of the following statements is/are
Q82: Acetic acid, (HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>)is a weak acid
Q88: Calculate the total number of unpaired electrons
Q99: What are the products of the chlor-alkali
Q134: In cation-exchange resins,what ion replaces Ca<sup>2+</sup> and
Q135: Choose the species that is the most