Which of the following result(s) in an increase in the entropy of the system?
I. 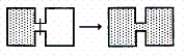
II.
Br2(g) Br2(l)
III.
NaBr(s) Na+(aq) + Br-(aq)
IV.
O2(298 K) O2(373 K)
V.
NH3(1 atm,298 K) NH3(3 atm,298 K)
Definitions:
First-degree Murder
A crime involving premeditation or planning before killing another person, considered one of the most serious forms of homicide.
Armed Robbery
A criminal act involving theft or attempted theft under threat of violence, where the perpetrator is armed with a weapon.
Tax Fraud
The illegal act of evading or defeating tax laws, often involving the deliberate falsification of tax returns to reduce tax liability.
Violates An Injunction
Engaging in an action that disobeys a court's legally enforceable order or ruling, typically resulting in legal penalties.
Q23: A titration of 200.0 mL of
Q53: Electron capture transforms <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="Electron capture
Q65: Which of the following pairs is incorrect?<br>A)iodine
Q89: Which of the following will not produce
Q89: Which of the following metals has the
Q91: For the cell Cu(s)| Cu<sup>2+</sup> || Ag<sup>+</sup>
Q94: Which of the following species cannot act
Q100: What would be the effect on the
Q102: The pH of a 0.150 M solution
Q116: As water is heated,its pH decreases.This means