When a stable diatomic molecule spontaneously forms from its atoms,what are the signs of H°, S°,and G°? 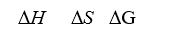
Definitions:
Crucial Experiment
An experiment that is critically important in testing and potentially proving or disproving a hypothesis or theory.
Hypothesis
A hypothesis is a proposed explanation for a phenomenon, serving as a starting point for further investigation to be tested through experimentation or observation.
Investigative Method
A systematic approach employed in various disciplines to explore and gather facts or information about a particular subject, phenomenon, or aspect of study.
Resources
Assets, material or nonmaterial, that individuals or groups have access to and can use for support, survival, or to achieve goals.
Q28: The enthalpy of vaporization of ammonia
Q38: Suppose a buffer solution is made
Q46: How many moles of HCl need
Q64: The following question refers to a
Q70: The following question refers to the
Q78: Consider the reaction represented by the equation
Q91: Lithium metal with excess O<sub>2</sub>(g).
Q94: Consider the titration of 300.0 mL
Q133: The most important hydride of nitrogen is<br>A)ammonia<br>B)hydrazine<br>C)styrofoam<br>D)agricultural
Q142: Consider the following reaction: AgBr(s)+ 2CN<sup>-</sup>(aq)