The acid dissociation constant for a weak acid HX at 25°C is 4.3 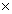 10-8.Calculate the free energy of formation for X-(aq) at 25°C.The standard free energies of HX(aq) and H+(aq) at 25°C are -208.3 kJ/mol and 0,respectively.
10-8.Calculate the free energy of formation for X-(aq) at 25°C.The standard free energies of HX(aq) and H+(aq) at 25°C are -208.3 kJ/mol and 0,respectively.
Definitions:
Disadvantages
The negative aspects or drawbacks associated with a situation, decision, or action.
Parallel Construction
A grammatical and stylistic approach in writing where similar parts of a sentence are grammatically the same, or similar in construction.
Lists
Collections of items, data, or information organized sequentially or categorically for reference or management.
Business Documents
Documents that are used in business environments, including memos, reports, business plans, and financial statements.
Q8: Which of the following atomic symbols is
Q10: A 10.0-g sample of solid NH<sub>4</sub>Cl is
Q12: Consider a solution containing the following cations:
Q27: Nuclides with too many neutrons to be
Q34: Calculate the pH of a 0.005 M
Q35: Calculate the pH of a 0.59
Q36: Which of the following solid salts should
Q47: The element with the widest liquid range
Q72: For a particular reaction in a
Q73: One milliliter (1.00 mL)of acid taken from