The acid dissociation constant for a weak acid HX at 25°C is 4.3 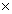 10-8.Calculate the free energy of formation for X-(aq) at 25°C.The standard free energies of HX(aq) and H+(aq) at 25°C are -208.3 kJ/mol and 0,respectively.
10-8.Calculate the free energy of formation for X-(aq) at 25°C.The standard free energies of HX(aq) and H+(aq) at 25°C are -208.3 kJ/mol and 0,respectively.
Definitions:
Interruption
The act of stopping or hindering a process or activity temporarily or permanently, causing a break in continuity.
Silent Patient
A patient who is either unwilling or unable to speak during therapy sessions, often posing challenges in diagnosis and treatment.
Withdrawn
A behavior characterized by a lack of interaction with other people, often due to depression, anxiety, or other mental health issues.
Suspicious
Characterized by or having a tendency to suspect, distrust, or question motives, often without evidence or justification.
Q12: Write the names of the following compounds:<br>a)<br>FeSO<sub>4</sub><br><sub>_</sub><br>b)<br>NaC<sub>2</sub>H<sub>3</sub>O<sub>2</sub><br><sub>_</sub><br>c)<br>KNO<sub>2</sub><br><sub>_</sub><br>d)<br>Ca(OH)<sub>2</sub><br><sub>_</sub><br>e)<br>NiCO<sub>3</sub><br><sub>_</sub>
Q22: Consider the reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="Consider the
Q50: The average rate of disappearance of ozone
Q53: The numerical value of the rate
Q73: Which of the following is the best
Q78: Consider the reaction represented by the equation
Q89: What volume of water must be
Q93: solid sodium nitrate (NaNO<sub>3</sub>)<br>A)acidic<br>B)basic<br>C)neutral<br>D)cannot tell<br>E)none of these
Q100: Which of these Group 2A elements reacts
Q111: Consider a solution made by mixing