Consider the gas phase reaction NO + 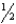 O2
O2  NO2 for which H° = -56.91 kJ and K = 1.46 106 at 25°C.
NO2 for which H° = -56.91 kJ and K = 1.46 106 at 25°C.
-Calculate H° at 25°C for the following reaction: 2NO + O2  2NO2
2NO2
Definitions:
Double-Declining-Balance Method
A method of accelerated depreciation where an asset's book value is reduced by twice the straight-line depreciation rate.
Depreciation Record
Depreciation Record is a detailed account of the depreciation amounts allocated over the life of an asset, which impacts the value of the asset on the balance sheet and the depreciation expense on the income statement.
150%-Declining-Balance
A method of accelerated depreciation, applying 150% of the straight-line rate to the declining balance of an asset, used to allocate larger depreciation expenses in the earlier years of an asset’s life.
Q4: The reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt=" The
Q28: SnI<sub>2</sub>
Q32: What is the molarity of a sodium
Q55: The first scientist to show that atoms
Q59: When the following reaction is balanced
Q60: For a certain process,at 300.K <span
Q72: All of the following are characteristics of
Q117: The hydrogen sulfate or bisulfate ion
Q134: Ammonium metavandate reacts with sulfur dioxide
Q148: Which of the following would produce a