A 50.0-mL sample of 0.100 M Ca(NO3) 2 is mixed with 50.00 mL of 0.200 M NaF.When the system has come to equilibrium,which of the following sets of conditions will hold? The Ksp for CaF2 is 4.0 10-11. Moles Solid CaF2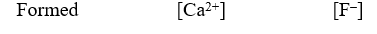
Definitions:
Negative Reference Group
A group an individual does not want to be associated with or emulate, affecting their behavior or choices.
Norms
Shared guidelines or rules within a group that dictate appropriate and expected behavior among members.
Social Agreements
Unwritten and often unspoken rules or understandings that govern the behavior of members within a society or group.
Beliefs
Convictions or acceptances that something exists or is true, especially without proof.
Q2: You are given a compound with the
Q24: Cl<sub>2</sub>O
Q40: NaH
Q40: A radioactive sample has an initial
Q52: A 50.00-mL solution of 0.0217 M
Q81: For the reaction CO(g)+ 2H<sub>2</sub>(g) <img
Q90: Calculate the pH of a 2.3
Q90: A chemical reaction that is first
Q99: The second law of thermodynamics states that:<br>A)The
Q120: solid ammonium acetate (NH<sub>4</sub>C<sub>2</sub>H<sub>3</sub>O<sub>2</sub>).For NH<sub>4</sub><sup>+</sup>,K<sub>a</sub> =