Consider the Following Information About the Diprotic Acid,ascorbic Acid 10-5)
HAs- As2- + H+ pKa
Consider the following information about the diprotic acid,ascorbic acid.(H2As for short,molar mass 176.1)
H2As  HAs- + H+ pKa
HAs- + H+ pKa
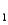 = 4.10 (Ka
= 4.10 (Ka
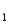 = 7.9 10-5)
= 7.9 10-5)
HAs-  As2- + H+ pKa
As2- + H+ pKa
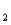 = 11.79 (Ka
= 11.79 (Ka
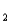 = 1.6 10-12)
= 1.6 10-12)
The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below: 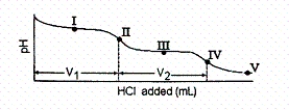
-What is the pH at point III?
Definitions:
Recessionary Gap
A situation in macroeconomics when an economy operates below its full-employment equilibrium, leading to a gap in output.
Government Spending
The total amount of public expenditure by government entities on goods, services, and public projects.
Automatic Stabilizers
Economic policies and programs designed to offset fluctuations in a nation's economic activity without intervention by the government or policymakers, such as unemployment insurance and progressive taxation.
National Income
The total value of all goods and services produced by a country over a specific time period, adjusted for net income from foreign investments.
Q12: Radioactive tracers are useful in studying
Q14: Calculate the osmotic pressure (in torr)of
Q21: What is the value of n?<br>A)0<br>B)0.5<br>C)1<br>D)1.5<br>E)2
Q33: The half-life is constant.<br>A)zero order in A<br>B)first
Q38: Of <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q40: Find the value of the equilibrium
Q53: The solubility of AgCl in water is
Q67: The standard molar free energies of
Q68: When the equation for the following
Q75: A strip of copper is placed