Consider the Following Information About the Diprotic Acid,ascorbic Acid 10-5)
HAs- As2- + H+ pKa
Consider the following information about the diprotic acid,ascorbic acid.(H2As for short,molar mass 176.1)
H2As  HAs- + H+ pKa
HAs- + H+ pKa
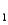 = 4.10 (Ka
= 4.10 (Ka
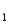 = 7.9 10-5)
= 7.9 10-5)
HAs-  As2- + H+ pKa
As2- + H+ pKa
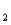 = 11.79 (Ka
= 11.79 (Ka
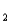 = 1.6 10-12)
= 1.6 10-12)
The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below: 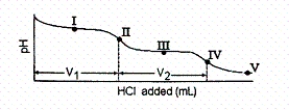
-What is the pH at point I (V1/2 HCl added) ?
Definitions:
Q3: A 100-mL sample of water is
Q4: For a certain process at 355
Q19: The overall K<sub>f</sub> for the complex
Q67: The solubility of O<sub>2</sub> in water
Q69: The elementary chemical reaction O +
Q75: The lead-208 nucleus has a mass
Q85: A galvanic cell consists of
Q98: What is the order of this reaction?<br>A)3<br>B)2<br>C)1<br>D)0<br>E)cannot
Q105: Given that <span class="ql-formula" data-value="\Delta"><span
Q112: What is the value of m?<br>A)0<br>B)0.5<br>C)1<br>D)1.5<br>E)2