Consider the Following Information About the Diprotic Acid,ascorbic Acid 10-5)
HAs- As2- + H+ pKa
Consider the following information about the diprotic acid,ascorbic acid.(H2As for short,molar mass 176.1)
H2As  HAs- + H+ pKa
HAs- + H+ pKa
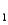 = 4.10 (Ka
= 4.10 (Ka
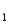 = 7.9 10-5)
= 7.9 10-5)
HAs-  As2- + H+ pKa
As2- + H+ pKa
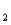 = 11.79 (Ka
= 11.79 (Ka
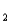 = 1.6 10-12)
= 1.6 10-12)
The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below: 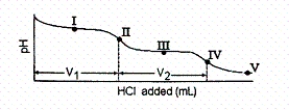
-What is the pH at point III?
Definitions:
Salt Dome
A geological formation created when layers of salt are pushed upward by the weight of overlying rock, sometimes trapping oil and gas.
Fault Zone
A region of numerous, closely spaced faults where the Earth's crust has fractured and moved.
Unconformity
A gap in the geological record where rock layers have been eroded or never deposited, representing a period of non-deposition or erosion.
Energy Resource
Any natural or artificial substance or phenomenon that can be utilized to produce power, such as fossil fuels, solar, wind, or hydro power.
Q21: Barium carbonate has a measured solubility
Q29: You have a 250.-mL sample of
Q35: For the equilibrium system: CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img
Q44: A 25.00-mL sample of propanoic acid,CH<sub>3</sub>CH<sub>2</sub>COOH,of unknown
Q45: What is the percent by mass of
Q69: Calculate the pH of the following aqueous
Q92: What quantity of charge is required to
Q96: Determine the magnitude of the frequency
Q96: How many faradays are involved in conversion
Q101: A plot of [A] vs.t is a