If,at a given temperature,the equilibrium constant for the reaction H2(g) + Cl2(g)  2HCl(g) is Kp,then the equilibrium constant for the reaction HCl(g)
2HCl(g) is Kp,then the equilibrium constant for the reaction HCl(g) 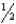 H2(g) +
H2(g) + 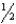 Cl2 (g) can be represented as:
Cl2 (g) can be represented as:
Definitions:
Commercial Paper
An unsecured, short-term debt instrument issued by corporations, typically used for the financing of accounts receivable, inventories, and meeting short-term liabilities.
Bank Loan
A sum of money lent by a bank to a borrower at an interest rate, which is to be repaid with interest according to the terms of the loan agreement.
Interest Rate
The percent of principal charged by the lender for the use of its money.
Prime Rate
The interest rate that commercial banks charge their most creditworthy customers, often used as a benchmark in lending.
Q32: What is the molarity of a sodium
Q44: Given the equilibrium constants for the following
Q59: At a particular temperature,N<sub>2</sub>O<sub>5</sub> decomposes according
Q60: Which of the following compounds has
Q87: Which of the following favors the solubility
Q88: The reaction 2H<sub>2</sub>O(g) <span class="ql-formula" data-value="\to"><span
Q93: The solubility of a gas usually increases
Q101: How many moles of electrons are
Q109: The rate constant for a reaction
Q125: The pH of a 1.0 M aqueous