The Decomposition of N2O5(g)to NO2(g)and O2(g)obeys First-Order Kinetics 10-5 S-1 at 25°C,what Is the Half-Life for the Reaction
The decomposition of N2O5(g) to NO2(g) and O2(g) obeys first-order kinetics.Assuming the form of the rate law is: 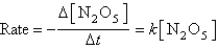
Where k = 5.4 10-5 s-1 at 25°C,what is the half-life for the reaction described?
Definitions:
Gender and Leadership
The study and analysis of how gender identity and roles impact leadership styles, effectiveness, and opportunities in organizations.
Interactive Leadership
A leadership style characterized by open communication and active engagement with team members to inspire and motivate.
Transactional Approach
A leadership model focusing on the exchanges or transactions between leaders and followers, often involving rewards for performance.
Democratic
A leadership or government style characterized by the principle of making decisions by majority vote, often emphasizing equality and participation.
Q2: What will happen if a small amount
Q19: Calculate the pH of a solution
Q40: A solution contains 10.mmol of H<sub>3</sub>PO<sub>4</sub>
Q67: For a certain reaction at 25.0°C,the
Q80: What are the units for the rate
Q81: Express the number <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="Express the
Q88: Which of these statements is false?<br>A)Diamond is
Q92: What major species is (are)present at point
Q102: The vapor pressure of water at 80°C
Q142: Describe the surface whose equation in