Multiple Choice
Below is a phase diagram for compound Q.You wish to purify a sample of Q that was collected at P = 1.0 atm and T = 100 K by subliming it.In order to sublime the sample,you should: 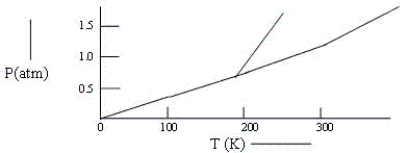
Definitions:
Related Questions
Q28: What volume of a 0.771 M solution
Q44: Find a unit vector that has the
Q58: A crystal was analyzed with x-rays having
Q74: Nitrogen gas (N<sub>2</sub>)reacts with hydrogen gas (H<sub>2</sub>)to
Q83: A quantitative observation<br>A)contains a number and a
Q98: Silver chloride crystallizes with the sodium chloride
Q103: The half-life for this experiment is<br>A)1.11
Q154: Describe the vertical traces x =
Q220: Consider the solid sphere <span
Q260: Convert <span class="ql-formula" data-value="( 1