A sample consisting of CO2(g) and CO2(s) at equilibrium at -78°C and 1 atm pressure is heated to -30°C and the pressure is increased to 8 atm.Based on the phase diagram below,what will happen? 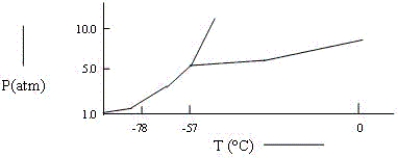
Definitions:
Gender-oriented
Pertaining to the customization of products, services, or marketing messages based on gender differences and preferences.
Sex-typed
Refers to characteristics or behaviors that are traditionally associated with one gender over the other.
Gender-bending
The act of defying or questioning traditional gender roles through one's actions, appearance, or demeanor.
Extended Self
A concept in consumer behavior that suggests individuals' possessions and their associations significantly contribute to and reflect their identities.
Q2: The reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt=" The
Q3: Find the intersection point of the
Q7: In which of the following processes will
Q73: Which is the strongest acid of the
Q74: The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys
Q109: The rate constant for a reaction
Q220: Consider the solid sphere <span
Q233: Convert <span class="ql-formula" data-value="\left( 2
Q239: Describe the surface whose equation in
Q240: Sketch and identify the quadric surface