A sample consisting of CO2(g) and CO2(s) at equilibrium at -78°C and 1 atm pressure is heated to -30°C and the pressure is increased to 8 atm.Based on the phase diagram below,what will happen? 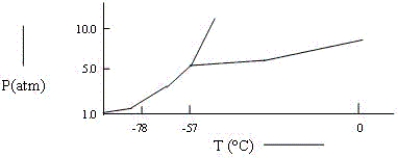
Definitions:
Perfect Tender Rule
A legal principle in commercial law that sellers must deliver goods that exactly meet the terms of the contract without any deviation.
Material Breach
A substantial breach of a significant term or terms of a contract that excuses the nonbreaching party from further performance under the contract and gives the nonbreaching party the right to recover damages.
Partially Destroyed
Refers to property or goods that have been damaged but not completely ruined, retaining some value or use.
Contract Price
The price agreed upon by the contracting parties as the amount to be paid for the fulfillment of the contract obligations.
Q25: Determine whether the points A
Q39: Define amphoteric substance.
Q41: The average value for the rate
Q48: Consider pure water separated from an aqueous
Q70: Which of the following would you expect
Q100: We can predict the solubility of a
Q156: Describe the surface whose equation in
Q179: Find the volume of the parallelepiped with
Q224: Let <span class="ql-formula" data-value="f (
Q242: Find the length of the vector