Tooth enamel is composed of the mineral hydroxyapatite. It is essentially insoluble in water with Ksp 2.3 1059, but it reacts with weak acids in the mouth as described by one of the reaction equations below. You can determine the equilibrium constant, K, for this reaction from Ksp and Kw (the equilibrium constant for water autoionization, Kw 1.0 1014) . What is the value of K? 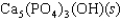
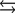
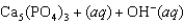
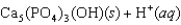
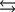
Definitions:
Canal Portion
A segment of a canal or other similar structure that is distinguished for specific reference or analysis.
Sensory Input
Information that the brain receives via the senses, including sight, sound, smell, taste, and touch, which is then processed to guide responses and actions.
Blood Vessels
Tubular structures in the body through which blood flows, including arteries, veins, and capillaries.
Depth of Respirations
Refers to the degree of chest or abdominal expansion during breathing, indicating the volume of air inhaled or exhaled.
Q12: Which of the following is not true
Q14: The anticodon loop of the first tRNA
Q37: Eukaryotic sexual life cycles show tremendous variation.
Q42: Consider two complex ions: (I) [Co(CN)<sub>6</sub>]<sup>3</sup><font face="symbol"><sup></sup></font>,
Q52: If a homozygous bent wing fly is
Q67: Which metal, by the number of atoms,
Q74: Cell membranes are formed by _<br>A)a lipid
Q98: Which statement does not correctly describe a
Q109: The correct formula for ammonium tetracyanoplatinate(II) is
Q117: Draw the structure of cisplatin and briefly