Different concentrations of ions on either side of a cell membrane constitute a concentration cell, which is described by the Nernst equation given below. What is the electrochemical potential across a membrane when the sodium ion concentration is 0.10 M on one side of the membrane and 0.010 M on the other side, and the temperature is 37C (body temperature) ? (Given: 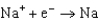 ,
, 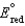 2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K) )
2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K) )
E E - 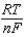 InQ
InQ
Definitions:
Invoice
A document issued by a seller to a buyer that lists the products or services provided and the amount due for payment.
Netiquette
The set of rules and conventions governing respectful and polite behavior in online communication.
Flaming
The equivalent of a verbal lashing on the Internet.
FOB Shipping Point
A shipping term indicating that the buyer assumes responsibility for the goods and their transportation costs from the seller's location.
Q2: What is meant by the description "antiparallel"
Q10: Examine the table of codons in Figure
Q15: Which component is not directly involved in
Q17: Which of the following statements best describes
Q28: Which of the following is the smallest
Q36: Which one of the following statements is
Q43: Which one of the following molecules is
Q53: Which of the following statements best describes
Q160: An electrochemical cell is constructed with a
Q161: A barrel of oil produces about 5.9