Different concentrations of ions on either side of a cell membrane constitute a concentration cell, which is described by the Nernst equation given below. A cell must expend energy to maintain this concentration gradient by moving ions from one side of the membrane to the other. How much energy must be expended to transport 1 mole of sodium ions across a membrane when the sodium ion concentration is 0.10 M on one side of the membrane and 0.010 M on the other side, and the temperature is 37C (body temperature)?
(Given: 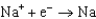 ,
, 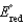 2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K))
2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K))
E E - 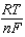 InQ
InQ
Definitions:
Oxygen
A chemical element essential for aerobic respiration in living organisms, playing a crucial role in energy production within cells.
Carbon
A chemical element with symbol C and atomic number 6, known as the basis of all known life and the fourth most abundant element in the universe by mass.
Hydrogen
The lightest and most abundant element in the universe, consisting of one proton and one electron, and widely used in chemical reactions and as a potential clean energy source.
Microglia
Specialized glial cells in the brain and spinal cord that act as macrophages, cleaning up debris and dead cells.
Q10: Electrochemical cell potentials can be used to
Q44: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 13.3 Which
Q46: In the reaction below, _ is the
Q48: Tooth enamel is composed of the mineral
Q84: Vanadium is an ultratrace metal in the
Q105: The unit of current, ampere (A), is
Q111: Phosphorus-32 is a radioactive isotope used as
Q113: Which one of the following statements about
Q127: When an atom of uranium-235 captures a
Q144: It is interesting to compare the energy