Use the table of standard reduction potentials below to identify the metal or metal ion that is the strongest reducing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 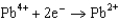 1.80
1.80 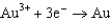 1.50
1.50 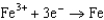 0.771
0.771 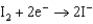 0.535
0.535 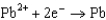 0.124
0.124 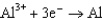 1.66
1.66 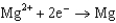 2.37
2.37 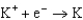 2.93
2.93
Definitions:
Chart of Account Order
The systematic arrangement of accounts within a chart, typically organized by type and number.
Worksheet
A paper or electronic document used by accountants to compile and analyze financial data ahead of the preparation of financial statements.
Permanent Accounting Record
A permanent accounting record is a documented history of a company's financial transactions that are kept for long-term reference and compliance purposes.
General Ledger
A comprehensive record of all financial transactions that occur within a company, serving as the primary source for preparing financial statements.
Q15: In a titration of monoprotic acids and
Q20: Given the following two measurements of the
Q21: The bonds between the zinc ion and
Q40: Write the reaction equation and the equilibrium
Q76: Calculate the nuclear binding energy per nucleon
Q95: Reduction is the _<br>A)gain of electrons.<br>B)loss of
Q129: An oligopeptide consists of up to _
Q142: Describe the structural features of an <font
Q145: The dominant mechanism through which radiation damages
Q153: Which statement is not correct for a