Use the table of standard reduction potentials below to identify the metal or metal ion that is the strongest oxidizing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 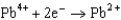 1.80
1.80 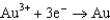 1.50
1.50 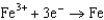 0.771
0.771 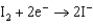 0.535
0.535 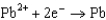 0.124
0.124 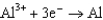 1.66
1.66 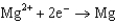 2.37
2.37 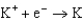 2.93
2.93
Definitions:
Publishers
Companies or individuals that prepare and issue books, journals, music, or other works for sale.
Household Chores
Routine tasks and activities performed in managing a home, such as cleaning, cooking, and maintenance.
Incompatibility
The state of being unable to coexist in harmony due to differences in behaviors, beliefs, or systems.
Bigamy
The act of marrying someone while already being married to another person, which is illegal in many jurisdictions.
Q16: Draw the d orbital splitting diagram for
Q23: Chromium is an ultratrace metal in the
Q44: The diagram below represents a voltaic cell.
Q56: What constitutes a standard hydrogen electrode?
Q101: Draw the d orbital splitting diagram for
Q117: Which cell diagram is correct for this
Q131: Which one of the following is a
Q134: When you increase the volume of a
Q137: Exposure to ionizing radiation usually is measured
Q143: What is the value of the equilibrium