Use the table of standard reduction potentials below to identify the metal or metal ion that is the weakest oxidizing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 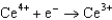 1.70
1.70 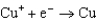 0.520
0.520 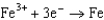 0.036
0.036 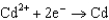 0.400
0.400 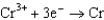 0.730
0.730 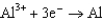 1.66
1.66
Definitions:
Criticism
The expression of disapproval or the identification of faults in someone or something based on perceived mistakes or weaknesses.
Unified Communication
A system that integrates various communication methods within a business, such as voice, video, messaging, and email, into a single interface.
Mobile Communication
The exchange of information through mobile devices, such as smartphones and tablets, allowing for communication regardless of location.
Collaboration
The action of working with someone to produce or create something.
Q10: Magnesium is found in which one of
Q13: Transcription is the process of _<br>A)copying DNA
Q90: Sodium carbonate is produced using the Solvay
Q103: Balance the following nuclear equation by supplying
Q110: Cobalt-56 decays by emitting a positron. What
Q123: Of the three modes of molecular motion-vibration,
Q125: If the free-energy change of the following
Q133: What kind of a bond connects simple
Q137: Exposure to ionizing radiation usually is measured
Q140: Which type of radiation has the lowest