Use the table of standard reduction potentials below to identify the metal or metal ion that is the weakest reducing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 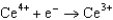 1.70
1.70 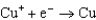 0.520
0.520  0.036
0.036 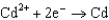 0.400
0.400 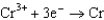 0.73
0.73 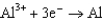 1.66
1.66
Definitions:
Oligopoly
A market structure characterized by a small number of firms dominating the market, leading to limited competition.
Differentiated Products
Goods or services that are distinguished from each other by characteristics like quality, design, branding, etc., making them not perfectly substitutable.
Interdependence
A situation in which the production processes or outcomes of different firms, sectors, or countries are dependent on each other.
Oligopoly
A market structure characterized by a small number of firms whose behavior is interdependent.
Q16: Hearing aid batteries utilize a zinc/air electrochemical
Q22: The equilibrium constant for a reaction is
Q37: The release of radioactive cesium-137 into the
Q49: The concentration of an ultratrace essential element
Q54: Using the thermodynamic data below, determine the
Q87: Plants react urea with water to produce
Q92: Which statement about chirality is not correct?<br>A)A
Q116: Fluorine-18 is an isotope commonly used in
Q128: Uranium-235 enrichment involves separations of isotopes of
Q166: If 3.500 g of Ni are reacted