Consider the following standard reduction potentials. Reduction Half-Reaction 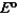 (volts)
(volts) 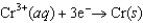 0.74
0.74 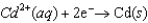 0.40
0.40 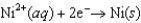 0.23
0.23
The Cr/Cr3 half-reaction can be paired with the other two to produce voltaic cells because ________
Definitions:
PLC Sequencer
A PLC (Programmable Logic Controller) function that executes operations or tasks in a predefined, sequential order for process control.
Transferred Information
Data or signals that have been moved or conveyed from one system, component, or device to another.
Sequencer Instruction
A programmable logic controller instruction used to execute a sequence of events or operations in a specific order.
Output Module
An output module is a component in a control system that interfaces the controller with the output devices, facilitating control actions.
Q28: Plants convert nitrogen into ammonia. This biological
Q30: Which base is not present in RNA?<br>A)adenine<br>B)cytosine<br>C)guanine<br>D)thymine<br>E)uracil
Q33: Radon-222 has a half-life of 3.8 days.
Q49: The capacity of a battery usually is
Q61: Which of the following is the best
Q69: If 15 g of aluminum from an
Q83: Copper is a trace metal in the
Q130: Fluorine-18 is a radioactive isotope used as
Q143: Which statement about corrosion is not correct?<br>A)Corrosion
Q152: The molecule drawn below is an example