Based on the information in the table of standard reduction potentials below, what is the standard cell potential for an electrochemical cell that has iron, Fe, and magnesium, Mg, electrodes? Also, identify the cathode. Standard Reduction
Potentials (volts) in Aqueous Solution 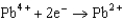 1.80
1.80 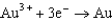 1.50
1.50 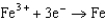 0.771
0.771 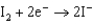 0.535
0.535 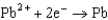 0.124
0.124 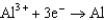 1.66
1.66 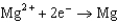 2.37
2.37 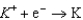 2.93
2.93
Definitions:
Phylum Arthropoda
The largest phylum in the animal kingdom, including insects, arachnids, myriapods, and crustaceans, characterized by segmented bodies and jointed limbs.
Jointed Appendages
Characteristic features of arthropods, including insects and crustaceans, where limbs are jointed, allowing for movement.
Planarians
Flatworms belonging to the Turbellaria class, known for their remarkable regenerative ability and as subjects of biological research.
Circulatory System
The body system that functions in internal transport and protects the body from disease.
Q8: What is the standard entropy change when
Q18: Butanoic acid contributes to the rancid odor
Q20: A phosphate buffer solution (25.00 mL sample)
Q37: Which one of the following molecules is
Q59: The concentration of a major essential element
Q62: Superoxide dismutase is an enzyme that converts
Q85: Identify how the bases and sugars that
Q102: Which statement characterizes the following table? Temperature
Q134: What is the change in free energy
Q139: Which process converts a neutron into a