Using the following data, determine the standard cell potential 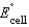 for the electrochemical cell constructed using the following reaction.
for the electrochemical cell constructed using the following reaction. 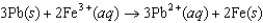 Half-reaction Standard Reduction Potential (V )
Half-reaction Standard Reduction Potential (V ) 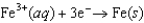 0.771
0.771 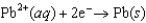 0.124
0.124
Definitions:
Classical Theory
in economics, refers to a school of thought that emphasizes free markets, the role of competition, and the minimal intervention of government in the economy, foundational to later economic theories.
Wages And Prices
The relationship and dynamics between the compensation paid to labor and the cost of goods and services in an economy.
Full Employment
A situation in an economy where all available labor resources are being used in the most efficient way possible, typically reflected by the absence of cyclical unemployment.
Keynes
Relates to John Maynard Keynes, a UK-based economist whose notions significantly altered macroeconomics theory and the financial policies of governments.
Q7: The tricarboxylic acid cycle (a.k.a. Krebs cycle)
Q26: Explain how a buffer solution manages to
Q41: Because of recent advances in recovery technology,
Q50: Hearing aid batteries utilize a zinc/air electrochemical
Q52: It is claimed that iron-56 is the
Q68: Beta emission is associated with _<br>A)conversion of
Q78: Alcohols for use as biofuels can be
Q89: A Lewis base is _<br>A)an electron-pair acceptor.<br>B)an
Q112: When pure water autoionizes, which ions are
Q112: Determine <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="Determine