Consider the voltaic cell based on the following reaction: 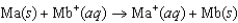 Two students are assigned to measure the standard cell potential. Yvonne claims that both solutions of ions have to be at exactly 1.00 M concentration, but Zelda is sure that the measurement will be the same if the concentrations of the two solutions are equal, but not necessarily 1.00 M. What do you think? (The temperature is controlled at 25C, so that's not an issue.)
Two students are assigned to measure the standard cell potential. Yvonne claims that both solutions of ions have to be at exactly 1.00 M concentration, but Zelda is sure that the measurement will be the same if the concentrations of the two solutions are equal, but not necessarily 1.00 M. What do you think? (The temperature is controlled at 25C, so that's not an issue.)
Definitions:
Soluble
The ability of a substance to dissolve in a solvent, forming a homogenous mixture at a specified temperature and pressure.
2-Butanone
A simple ketone with the formula CH3C(O)CH2CH3, also known as methyl ethyl ketone (MEK), used as a solvent.
Transformation
The process of changing the structure, composition, or form of a molecule or substance through chemical reactions.
Methylbutan-1-ol
A primary alcohol derived from butane by replacing one hydrogen atom with a hydroxyl group at the first carbon.
Q13: Transcription is the process of _<br>A)copying DNA
Q20: The Mg<sup>2</sup><font face="symbol"><sup></sup></font> ion plays a significant
Q22: Why are higher temperatures necessary for fusion
Q26: The molecule drawn below is an example
Q30: The heaviest stable nucleus is an isotope
Q34: Identify two processes that convert a proton
Q35: How many different nucleic acid bases are
Q59: Which of the following states of motion
Q121: Particles emitted in radioactive decay have different
Q129: An oligopeptide consists of up to _