A concentration cell is constructed by using the same half-reaction for both the cathode and anode. What is the value of Ecell for a concentration cell that combines silver electrodes in contact with 0.10 M silver nitrate and 0.00003 M silver nitrate solutions? ( 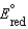 0.80 V for Ag/Ag)
0.80 V for Ag/Ag)
Definitions:
Income Taxes
Taxes imposed by the government directly on income, both earned (salaries, wages, commissions) and unearned (interest, dividends).
Indirect Quote
A foreign exchange rate quotation that specifies the foreign currency amount that can be purchased with one unit of the domestic currency.
Country B's Currency
The legal tender or monetary system used in a hypothetical Country B, which can be exchanged for goods and services or traded for other currencies.
Country A's Currency
The legal tender issued by Country A's central bank or monetary authority, used as a medium of exchange within Country A.
Q17: For each of the bonds in the
Q31: A cup of coffee has a hydroxide
Q38: Aqueous solutions of many metal cations have
Q52: Plants convert nitrogen into ammonia. This biological
Q64: Which statement about a voltaic cell is
Q75: Which statement A-D is not correct? Pure
Q81: Which of the following statements is/are correct?
Q82: Calcium is a major essential element in
Q109: What is the hydronium ion concentration of
Q111: Which ending to the statement is not