From the following table of standard reduction potentials, identify the best oxidizing and reducing agents. Also, determine the species from the table that is(are) capable of oxidizing iron to iron(III).
Standard Reduction
Potentials (volts) in Aqueous Solution 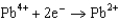 1.80
1.80 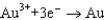 1.50
1.50 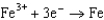 0.771
0.771  0.535
0.535 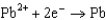 0.124
0.124 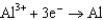 1.66
1.66 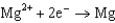 2.37
2.37 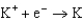 2.93
2.93
Definitions:
Followers Respect
The admiration and esteem that followers have for their leader, often based on the leader's qualities, actions, and integrity.
Reevaluate Perspectives
The process of reassessing and potentially altering one’s viewpoints or beliefs based on new information or changes in context.
Inspirational Motivation
The ability to inspire and motivate individuals or teams to achieve high levels of performance and commitment through a compelling vision of the future.
Transformational Leader
A type of leadership where the leader works with teams to identify needed change, creating a vision to guide the change through inspiration and executing the change in tandem with committed members of the group.
Q3: The entropy of fusion for ice at
Q4: The equilibrium constant for a given reaction
Q5: The peak in nuclear binding energy/nucleon occurs
Q9: What is the K<sub>a</sub> for the pyridinium
Q54: Positron emission is associated with _<br>A)conversion of
Q56: What constitutes a standard hydrogen electrode?
Q84: Indometacin is a nonsteroidal antiinflammatory drug, often
Q90: Which statement A-D regarding a complex ion
Q113: In its linear form, which functional groups
Q180: The gas above the liquid in a