Using the following data, determine the standard cell potential 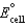 for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode.
for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode. 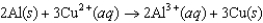 Half-reaction Standard Reduction Potential (V)
Half-reaction Standard Reduction Potential (V) 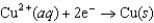 0.34
0.34 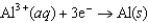 1.66
1.66
Definitions:
Direct Ownership
A form of business ownership where individuals or entities have complete control over their investments or assets without intermediaries.
Foreign Market
Refers to markets outside the company's home country where it can sell or market its products or services.
Exporting
The process of exporting products or services to a foreign country for the purpose of selling them.
Licensing
An agreement in which a company allows another entity to produce its product in exchange for a predetermined fee.
Q9: How many moles of sodium acetate must
Q15: What is K<sub>b1</sub> for the citrate ion,
Q21: Circle the glycosidinc linkage(s) in the following
Q35: Why is iodine-131 used effectively to treat
Q46: The most naturally abundant nuclides are those
Q64: Magnesium sulfate can be obtained at a
Q72: Boric acid frequently is used as an
Q88: Which process converts a proton into a
Q123: Of the three modes of molecular motion-vibration,
Q151: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>