Use the table of standard reduction potentials below to identify the metal or metal ion that is the strongest reducing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 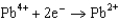 1.80
1.80 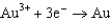 1.50
1.50 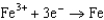 0.771
0.771 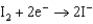 0.535
0.535 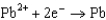 0.124
0.124 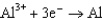 1.66
1.66 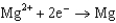 2.37
2.37 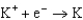 2.93
2.93
Definitions:
Dispositional Nature
Refers to inherent personality traits and tendencies that influence an individual's behavior and reactions.
Task Difficulty
The level of challenge presented by a task, which can affect an individual's performance and motivation.
Weiner
Refers to Bernard Weiner, an American psychologist known for his attribution theory in social psychology.
Expended Effort
The amount of energy, time, or resources utilized to achieve a result or complete a task.
Q24: Rank the following compounds from smallest to
Q31: What is the molar concentration of Ag<font
Q70: Ion channels in cell membranes control selective
Q99: Small traces of radioactive substances, mainly from
Q112: Determine <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="Determine
Q121: An amino acid is composed of _<br>A)ammonia
Q122: Dissolved concentrations of copper(II) as low as
Q133: What kind of a bond connects simple
Q160: The following reaction produces a peptide. Which
Q181: Which of the following processes is/are spontaneous?<br>I.