Use the table of standard reduction potentials below to identify the metal or metal ion that is the strongest oxidizing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 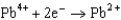 1.80
1.80 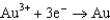 1.50
1.50 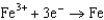 0.771
0.771 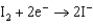 0.535
0.535 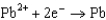 0.124
0.124 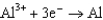 1.66
1.66 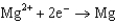 2.37
2.37 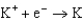 2.93
2.93
Definitions:
Revenue Recognition
The principle that determines the specific conditions under which revenue is recognized or accounted for.
SEC
The Securities and Exchange Commission, a U.S. government agency responsible for regulating and enforcing federal securities laws.
Persuasive Evidence
Information or documentation that reliably confirms the occurrence of a transaction or the reality of an asset or liability, meeting certain criteria to be recognized in financial statements.
Internet Companies
Entities primarily engaged in providing services or products via the internet, including e-commerce, social networking, and cloud computing.
Q8: A half-life is _<br>A)the life that a
Q21: The bonds between the zinc ion and
Q82: A Lewis acid is _<br>A)a proton donor.<br>B)a
Q84: Which of the relationships between the free-energy
Q102: Which statement is not correct? In hydrogen
Q107: A hydrogen fuel cell depends on _<br>A)fusion
Q122: The natural abundance of uranium-235 is _<br>A)a
Q137: Exposure to ionizing radiation usually is measured
Q150: The standard hydrogen electrode is _<br>A)used to
Q155: In 1989, two prominent electrochemists, Stanley Pons