Use the table of standard reduction potentials below to identify the metal or metal ion that is the weakest oxidizing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 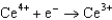 1.70
1.70 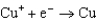 0.520
0.520 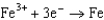 0.036
0.036 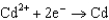 0.400
0.400 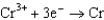 0.730
0.730 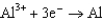 1.66
1.66
Definitions:
Gross Profit Inventory Method
An accounting method to estimate the value of ending inventory and cost of goods sold using the gross profit margin.
Inventory Valuation
The method used to determine the cost associated with an inventory at the end of an accounting period, impacting the cost of goods sold and net income.
Gross Profit
The financial metric indicating the difference between revenue and the cost of goods sold (COGS), reflecting the efficiency of core operations.
Consignor and Consignee
The consignor is the entity that owns goods being shipped, while the consignee is the entity receiving the goods for sale or safekeeping.
Q14: The symbol <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q29: Which of the following is the conjugate
Q49: The concentration of an ultratrace essential element
Q59: Which one of the following is not
Q71: Which graph below describes the decay of
Q71: Which statement describing the mechanisms by which
Q89: Which of the following is not a
Q100: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>
Q101: Extremely high temperatures are required to initiate
Q128: Uranium-235 enrichment involves separations of isotopes of