Consider the following standard reduction potentials. Reduction Half-Reaction 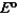 (volts)
(volts) 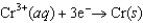 0.74
0.74 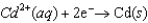 0.40
0.40 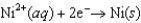 0.23
0.23
The Cr/Cr3 half-reaction can be paired with the other two to produce voltaic cells because ________
Definitions:
Insurance
A financial product sold by insurance companies to safeguard against the risk of financial losses, both big and small, that may result from damage to the insured or her property, or from liability for damage or injury caused to a third party.
Depreciation
The systematic allocation of the cost of a tangible asset over its useful life, reflecting the consumption of the asset's value over time.
Direct Materials Variances
The difference between the actual cost of direct materials used in production and the expected (standard) cost of those materials.
Fixed Overhead
Regular, unchanging costs incurred by a business, regardless of its level of production or sales, such as rent or salaries.
Q12: Which of the following is not true
Q25: Iodine's role is very specific. It is
Q29: Which of the following is the conjugate
Q35: Which of the following is the conjugate
Q45: Fluoride is added to toothpastes and drinking
Q72: What type of motion is depicted in
Q94: What is a microstate and how are
Q102: A substance that can act as both
Q113: Paxil (paroxetine) is a selective serotonin reuptake
Q119: Oleic acid, shown below, is _ <img