Based on the information in the table of standard reduction potentials below, what is the standard cell potential for an electrochemical cell that has chromium, Cr, and cadmium, Cd, electrodes? Also, identify the anode. Standard Reduction
Potentials (volts) in Aqueous Solution 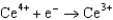 1.70
1.70 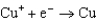 0.520
0.520 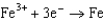 0.036
0.036 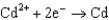 0.400
0.400 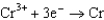 0.73
0.73 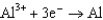 1.66
1.66
Definitions:
Equilibrium Quantity
The quantity of goods or services supplied and demanded at the equilibrium price, where the quantity supplied equals the quantity demanded.
Equilibrium Price
The price at which the quantity of a good or service demanded by consumers equals the quantity supplied by producers, resulting in market balance.
MP3 Players
Portable digital devices designed to play music files in the MP3 format, allowing users to listen to music on the go.
Equilibrium Quantity
The quantity of goods or services that is supplied and demanded at the equilibrium price, where the quantity demanded equals the quantity supplied.
Q4: A solution of nitrous acid (0.15 M,
Q29: A solution of hydrofluoric acid (0.27 M,
Q58: Tissue damage caused by radiation is measured
Q68: Three acids found in foods are lactic
Q78: What other particle is formed in the
Q79: For a chemical reaction that is not
Q88: A patient is injected with a 5.0
Q127: The standard entropy of N<sub>2</sub>(g) is 191.5
Q134: What is the change in free energy
Q158: The diagram below represents a voltaic cell.