Copper is oxidized by nitric acid. If this property were used in an electrochemical cell, what would the standard cell potential be? The relevant reduction reactions and standard reduction potentials are given below.0.34 V 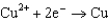 0.96 V
0.96 V 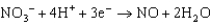
Definitions:
Estimated Date of Confinement (EDC)
The predicted date of a pregnant woman's delivery.
Glucose Tolerance Test
A medical test that measures the body's ability to metabolize glucose and is used to diagnose diabetes or prediabetes.
Intraepithelial Lesion
A precancerous change in the layer of cells found on the body's surface or lining internal organs, potentially leading to cancer if left untreated.
Malignancy
The presence of cancerous cells that have the ability to spread to other parts of the body and destroy healthy tissue.
Q36: The solubility of PbI<sub>2</sub> is 0.756 g
Q64: Which statement about a voltaic cell is
Q64: Radioactive iodine-125 and palladium-103 decay by X-ray
Q76: When [H<font face="symbol"><sup></sup></font>] <font face="symbol"></font> 4.0 <font
Q79: For a chemical reaction that is not
Q91: Uranium-238 decays to lead-206 through a series
Q101: You have a job as a summer
Q118: A peptide bond is the result of
Q122: Which type of noncovalent interaction supports the
Q162: Cobalt-60 decays to nickel-60. What particle is