The standard cell potential for the nickel-cadmium battery is 1.35 V, and the cell reaction can be written as 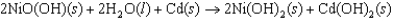 Which one of the following statements do you expect to be true based on the Nernst equation? Note Q reaction quotient, and K equilibrium constant for the cell reaction.
Which one of the following statements do you expect to be true based on the Nernst equation? Note Q reaction quotient, and K equilibrium constant for the cell reaction.
Definitions:
Culturally Defined
Aspects or norms that are determined and influenced by the culture in which they are found.
Non-verbal Meanings
Communication conveyed through means other than words, such as gestures, facial expressions, and body language.
Age
The length of time that a person or object has existed, often measured in years.
Height
The measurement of someone or something from base to top or from the ground to its highest point.
Q21: The activity of a radioactive sample is
Q23: Which of the following nuclides are most
Q48: An electrochemical cell at 298 K is
Q53: Determine the standard entropy of N<sub>2</sub>(g) given
Q78: What other particle is formed in the
Q84: Copper metal is purified by electrolysis. How
Q88: Which one of the following would make
Q118: A solution of nitrous acid (0.13 M,
Q128: Determine <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="Determine
Q134: When you increase the volume of a