A concentration cell is constructed by using the same half-reaction for both the cathode and anode. What is the value of Ecell for a concentration cell that combines silver electrodes in contact with 0.10 M silver nitrate and 0.00003 M silver nitrate solutions? ( 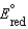 0.80 V for Ag/Ag)
0.80 V for Ag/Ag)
Definitions:
Single Plantwide Factory Overhead Rate
A method of allocating factory overhead to products based on a single, consistent rate applicable throughout the entire manufacturing plant.
Direct Labor Hours
The total hours worked by employees directly involved in the manufacturing process, often used as a basis for allocating factory overhead.
Desk Lamp Production
The process involving the assembly, testing, and packaging of desk lamps for consumer use.
Single Plantwide Factory Overhead Rate
A method of allocating total factory overhead costs to units produced, using a single rate for the entire manufacturing plant.
Q1: In the initial sequence of thorium-232 decay,
Q14: Determine the molar concentration of Cl<font face="symbol"><sup></sup></font>
Q29: Which of the following is not a
Q70: A typical D battery has a capacity
Q94: Which of the following molecules is/are chiral?
Q102: Which statement is not correct? In hydrogen
Q106: Which of the following are not amino
Q124: A typical AA battery has a capacity
Q137: For the following exothermic reaction, predict under
Q140: Values for <font face="symbol"></font>H<font face="symbol"></font> and <font