The oxidation of hydrogen by oxygen is one of the most-used reactions in fuel-cell technology. The overall reaction, which is given below, has a G value of 237 kJ/mol. What is the standard cell potential for this fuel cell? 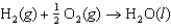
Definitions:
Oil Spill
The release of liquid petroleum hydrocarbon into the environment, particularly marine areas, due to human activities like drilling and transportation.
Environment Impact
The influence of human activities on the ecosystem, potentially causing habitat destruction, pollution, and climate change.
Spill Distance
The measure of how far a liquid or granular material can spread or disperse upon being spilled, often considered in environmental hazard planning.
Groundwater
Water found underground within the spaces between soil particles and cracks within rock structures.
Q41: Considering the tabulated values for the thermodynamic
Q42: What is the entropy change to the
Q45: The concentration of acetic acid (pK<sub>a</sub> <font
Q63: A 25.0 mL solution of quinine was
Q92: A Lewis acid is any species capable
Q98: At what temperature does the Fe(s) <img
Q124: Ranitidine, with a trade name of Zantac,
Q140: Which type of radiation has the lowest
Q158: Which statement is not correct? Starch and
Q165: Write nuclear reaction equations to show how