The oxidation of hydrogen by oxygen is one of the most-used reactions in fuel-cell technology. The overall reaction, which is given below, has a G value of 474 kJ/mol. What is the standard cell potential for this fuel cell? 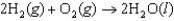
Definitions:
Units Received
Refers to the number of individual items or quantities of a product delivered to a recipient as part of a shipment or order.
Beginning Inventory
The worth of products ready to be sold at the beginning of a financial period.
Net Sales
The revenue generated from goods or services sold by a company after deducting returns, allowances, and discounts.
Markup
The difference between price and a seller’s cost of an item for sale. In dollars, it is the amount added to the cost of the goods in order to have a gross profit high enough to cover operating expenses and to make a net profit.
Q13: Calculate the K<sub>sp</sub> for Li<sub>3</sub>PO<sub>4 </sub>if at
Q30: Which base is not present in RNA?<br>A)adenine<br>B)cytosine<br>C)guanine<br>D)thymine<br>E)uracil
Q44: Which one of the following is not
Q54: Hearing aid batteries utilize a zinc/air electrochemical
Q59: Cobalt-60 is a radioactive isotope of Co
Q64: Magnesium sulfate can be obtained at a
Q88: If for a given chemical reaction at
Q98: At what temperature does the Fe(s) <img
Q103: Indicate which one of the following reactions
Q114: The unit of electrical power, watt (W),