The oxidation of methanol, as described by the equation below, has a G value of 937.9 kJ/mol. What is the standard cell potential for a methanol fuel cell? 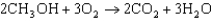
Definitions:
Age
The length of time that a person or organism has been alive; also a specific phase in life characterized by physical, psychological, or social attributes.
Compared
Evaluated or examined the similarities and differences between two or more entities.
Hypothesis
A proposed explanation for a phenomenon, made as a starting point for further investigation.
Schizophrenia
A long-term mental disorder involving a breakdown in the relation between thought, emotion, and behavior, leading to faulty perception, inappropriate actions and feelings, and a withdrawal from reality into fantasy and delusion.
Q21: The bonds between the zinc ion and
Q32: Which one of the following statements is
Q48: For each of the bonds in the
Q69: Which one of the following is not
Q72: Strontium-90 is most likely to decay by
Q75: In a spontaneous process, which of the
Q84: A solution with a pOH of 4.3
Q133: What kind of a bond connects simple
Q137: What is the linkage that forms between
Q150: Which one of the following is best