From the following table of standard reduction potentials, identify the best oxidizing and reducing agents. Also, determine the species from the table that is(are) capable of oxidizing iron to iron(III).
Standard Reduction
Potentials (volts) in Aqueous Solution 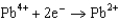 1.80
1.80 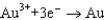 1.50
1.50 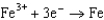 0.771
0.771  0.535
0.535 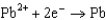 0.124
0.124 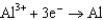 1.66
1.66 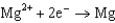 2.37
2.37 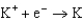 2.93
2.93
Definitions:
Marketing Objectives
Specific goals set by a business when promoting its products or services to potential consumers that should be achieved within a given time frame.
Opportunity
A favorable situation or condition that offers the prospect of a benefit, advantage, or solution to a problem.
SWOT Analysis
A strategic planning tool that identifies Strengths, Weaknesses, Opportunities, and Threats related to business competition or project planning.
Contingency Planning
The process of preparing for unforeseen events or emergencies by developing strategies in advance to handle and mitigate risks.
Q2: As the pH increases, the solubility of
Q29: Which of the following is not a
Q59: What is indicated by the shape of
Q83: Uranium-238 ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="Uranium-238 (
Q104: Which statement is not correct? A chiral
Q112: Determine <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="Determine
Q124: A typical AA battery has a capacity
Q128: Oxidation refers to _<br>A)an increase in oxidation
Q139: An electrochemical cell is constructed with a
Q140: Consider an electrochemical cell with a Zn